It smells like fresh-squeezed juice on Day 1.
But it smells like turpentine on Day 30.
We see this in almost every citrus formulation built on cold-pressed orange oil.
The problem is the breakdown.
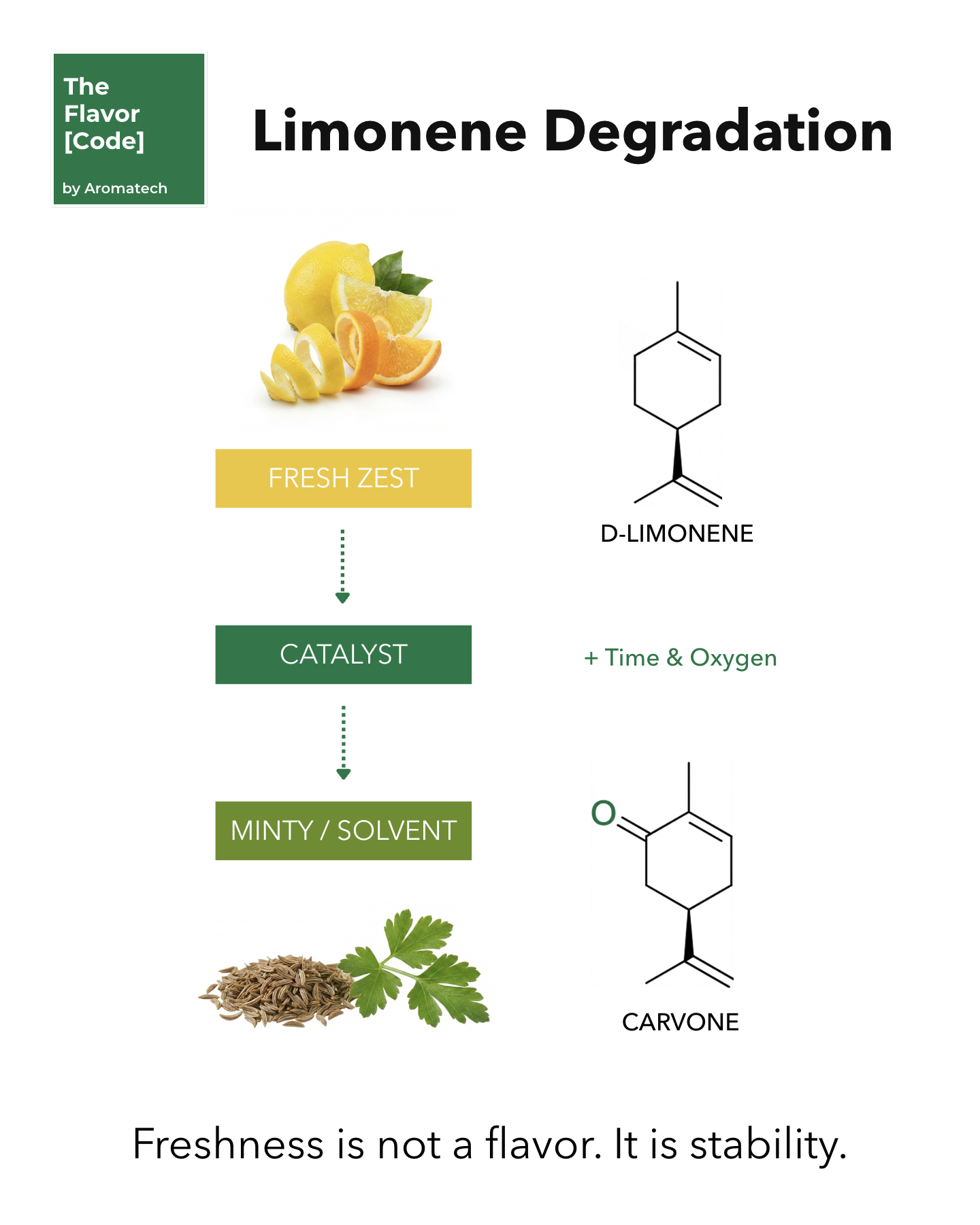
Limonene is chemically unstable.
It has two double bonds.
Light or heat triggers radical-mediated autoxidation.
And a low pH triggers acid-catalyzed hydration.
Both pathways destroy your flavor profile.
Pathway 1: Oxidation
Oxygen and metal ions form Limonene-6-hydroperoxide.
This is the silent phase.
It is odorless.
Then it decomposes into Carvone and Carveol.
Carvone tastes like caraway or mint.
Pathway 2: Hydration
Low pH forces an acid-catalyzed rearrangement.
Limonene becomes alpha-terpineol.
So your bright top note turns heavy and smells like turpentine.
Most developers try to fix this by adding more flavor.
That just increases the concentration of unstable compounds.
The solution is matrix management.
Control Dissolved Oxygen: Sparge your water. Otherwise, you start the reaction before capping the bottle.
Chelating Agents: Iron and copper catalyze radical formation. You must bind them.
Encapsulation: Hydration is inevitable at a low pH. You must physically separate the oil.
Freshness requires a complete lack of oxidation and hydration.